URGENT: Medical Device Recall Notice
Philips Respironics
CPAP and B--Level PAP Devices
The Philips Recall Notification
Those patients wishing to schedule a discussion regarding this matter can
Complete the contact form below to discuss options for care alternatives during this recall.
________________________________________________________________
As a Philips and ResMed provider, this page will be kept updated as information becomes more available.
PHILIPS MEDICAL DEVICE RECALL
Philips PAP Recall Notification
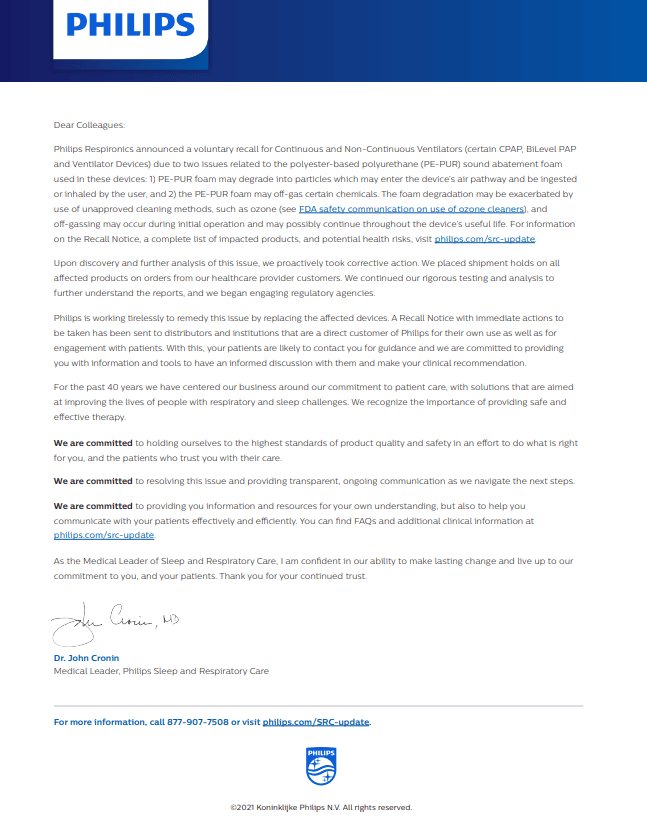
Most patients are unaware that on June 14, Philips Respironics issued a recall notification for an estimated 3-4 million bi-level PAP, CPAP and mechanical ventilator devices in response to potential health risks related to the sound abatement foam component in these devices. Philips is advising patients using affected BiLevel PAP and CPAP devices to discontinue use of their device and work with their provider to determine the most appropriate options for continued treatment. We are positioned to help you during this recall.
As a direct customer of Philips and ResMed and as an occasional distributer of PAP devices I received the URGENT Medical Device Recall Notice for distributors, institutions and device customers (patients). Also, as a DME provider of oral appliance therapy for OSA and having worked with physicians across the state I certainly have seen both forms of therapy and the advantages and disadvantages of each. Clearly with PAP we have efficacy but chase compliance while with Mandibular Advancement Devices we have compliance but chase efficacy. It goes without being said that no one form of therapy treats all segments of the population. The AADSM issued the following statement:
“Sleep apnea significantly impacts daytime sleepiness and quality of life, and many patients rely on CPAP as their form of therapy” “To ensure they continue to have treatment during this recall, those suffering from obstructive sleep apnea should know that Oral Appliance Therapy is a proven, effective treatment option.” First let us categorize and prioritize the population this recall effects.
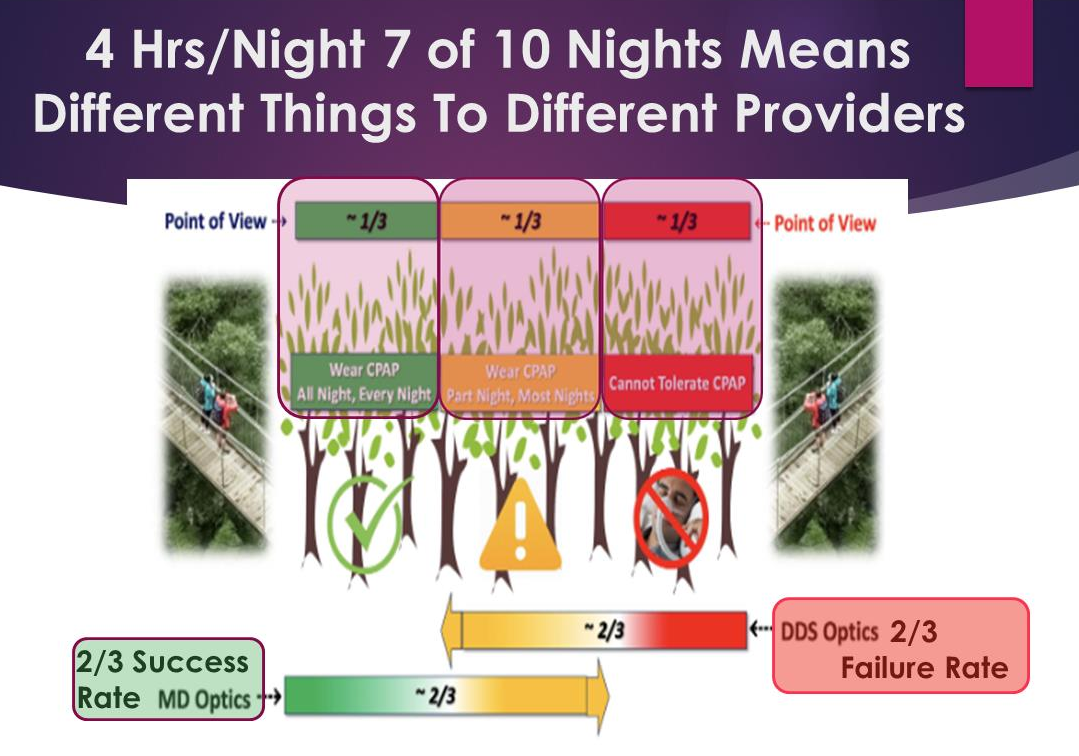
The following three groups exist:
Those wearing PAP all night
Those wearing PAP part of the night
Those that are PAP intolerant
Argument can be made that although 1/3 of patients are fully treated, 1/3 are sub-optimally treated (because PAP Compliance is considered to be only 4hrs/night 7 of 10 nights) and 1/3 are left untreated. Our medical system leaves all three groups largely unsupervised while the evidence suggests these three groups demand different considerations.
The Research Supports The Above
About The Philips Recall
About Oral Appliance Therapy
Updates